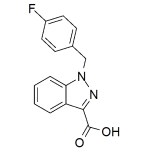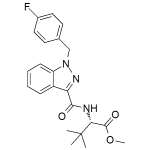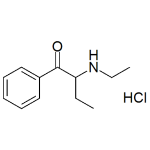Chemtos
Service, Quality, Speed
Chemtos
Service, Quality, Speed
Pyrovalerone Hydrochloride
High purity Pyrovalerone Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
Sisomicin Free Base
High purity Sisomicin Free Base includes a comprehensive Certificate of Analysis and all supporting analytical data
AB-FUBINACA metabolite 4 1mg/ml
High purity synthesized AB-FUBINACA metabolite 4 (MMB-FUBINACA metabolite 4) solution includes a comprehensive Certificate of Analysis and all supporting analytical data
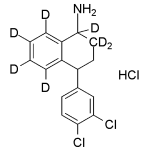
Norsertraline labeled d7 Hydrochloride
High purity Norsertraline-d7 Hydrochloride / N-Desmethylsertraline-d7 Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
TEG-carboxylate Labeled d4
High purity TEG-carboxylate Labeled d4 (TEG-carboxylic acid-d4, Triethylene glycol monocarboxylic acid labeled d4) includes a comprehensive Certificate of Analysis and all supporting analytical data
MDMB-FUBINACA 0.1mg/ml
High purity synthesized MDMB-FUBINACA (Methyl 2-(1-(4-fluorobenzyl)-1Hindazole-3-carboxamido)-3,3-dimethylbutanoate, US DEA C-I 7020) solution includes a comprehensive Certificate of Analysis and all supporting analytical data
N-Ethylbuphedrone HCl (NEB HCl)
High purity N-Ethylbuphedrone Hydrochloride (NEB HCl) includes a comprehensive Certificate of Analysis and all supporting analytical data
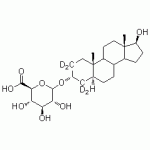
5α-Androstane-3α,17ß-diol-3-O-glucuronide-d4
High purity 5α-Androstane-3α,17ß-diol-3-O-glucuronide labeled d4 (Dihydroandrosterone-3-O-glucuronide labeled d4, 3α-Diol-G labeled d4, (3α,5α,17β)-17-Hydroxyandrostan-3-yl β-D-glucopyranuronate labeled d4, (3α,5α,17β)-17-Hydroxyandrostan-3-yl β-D-glucopyranuronate labeled d4) includes a comprehensive Certificate of Analysis and all supporting analytical data
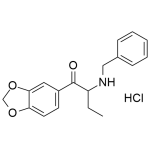
Benzylbutylone (BMDB) HCl 1mg/ml
High purity BMDB HCl (Benzylbutylone, 1-(1,3-benzodioxol-5yl)-2-(benzylamino)butan-1-one) solution includes a comprehensive Certificate of Analysis and all supporting analytical data